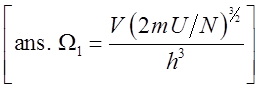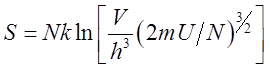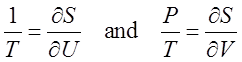|
Chaion Analytics |
Entropy
in Statistical Mechanics
Copyright ï³°an> 2011 by Robert Finkel |
||||||
|
ã°¡n style="font:7.0pt "Times New Roman"">
Probabilities ã°¡n style="font:7.0pt "Times New Roman"">
Averaging ã°¡n style="font:7.0pt "Times New Roman"">
Entropy ã°¡n style="font:7.0pt "Times New Roman"">
Moles &
Gas Constant ã°¡n style="font:7.0pt "Times New Roman"">
Thermodynamics ã°¡n style="font:7.0pt "Times New Roman"">
Partition
Function ã°¡n style="font:7.0pt "Times New Roman"">
Gibbs Entropy Copyright ï³°an>
2011 by Robert Finkel |
Entropy
is intimately connected to statistical concepts and is often one of the
functions most readily found from molecular models. Entropy S appears in the fundamental thermodynamic
expression Three forms of
entropy are widely used in thermal physics. The thermodynamic or classical
form is Most
traditional presentations of statistical mechanics begin by expressing
entropy in terms of W,
the number of equally probable microscopic states that constitute a given macroscopic
state. For example, a ᣲoscopic㴡te
where 2 people (molecules) are seated in any 6 chairs can be achieved in W = 30 飲oscopicê ways. Entropy
is a technical measure of randomness and this is apparent in the statistical
form. The entropy
Our
objective on this page is to use Eq.(1) to derive
state equations for ideal gas. A thermodynamic state equation like the
familiar De Broglie recognized that all matter exhibits both
wave and particle properties. The
expression deduced by de Broglie applies to all matter including the most familiar particles; photons,
electrons, protons, and neutrons. Each object is both a particle and a wave
and shows one property or the other depending upon the circumstances.
De Broglie expressed the wavelength l of the
matter-wave in terms of the momentum p
of the particle. Wavelength is Planck's constant h divided by p,.
You can use this derive some approximate expressions
for the number of microstates W in an ideal gas. While these are approximate, they
enable us to derive some correct state equations. This can be attributed to
the fact that unimportant approximate particulars are washed out by the averaging process
while the salient features survive to be reflected in the macroworld. A
crude measure of the number of microstates, denoted ã°¡n
style='font:7.0pt "Times New Roman"'> Evaluate microstates The
total number of microstates for N particles is the product of the of all
the independent individual microstates,
This
is not pretty, but it is easy to extract from it all possible macroscopic information
regarding ideal gas. We do this with
equations from thermodynamics involving partial
derivatives. Partial Derivatives Consider
a function S that depends on more
than one independent variable, say V
and U. We often need to
differentiate S with respect to one
variable, say V, while treating any
other independent variables (like U)
as if they are constants. This is the partial
derivative of S with respect to V
and is denoted as
ã°¡n
style='font:7.0pt "Times New Roman"'> Given a function
[ans. Ideal Gas Ideal
gas derivations are favorites to illustrate applications of statistical
mechanics. Two useful thermodynamic
equations are developed in our Thermodynamics
page. Here I present them as given:
ã°¡n
style='font:7.0pt "Times New Roman"'> Apply the thermodynamic equations
above to the entropy
|